Phosphates – it’s all in the chemistry
At Tessenderlo’s phosphate plant in Ham, in northern Belgium, phosphate rock is turned into a very pure dihydrated crystalline dicalcium phosphate. This isn’t the only method the group uses to make feed phosphates. Intimate knowledge of all available phosphates, claim the company’s experts, is the secret to good phosphorus nutrition for all species of livestock.
Animal nutrition does not work in a vacuum. Everything that goes into successful feeding, be it formulation, the quality of the raw materials, processing, additives, infection pressure, management techniques or genetics, has an influence on how a feed performs in the field. This even extends to the manufacturing process used to produce mineral feed ingredients. When Feed Mix visited the phosphate plant in Belgium recently, technical advisor Sjo Zwart and technical manager for feed ingredients, Dr. Wouter Bleukx explained how their intimate knowledge of the phosphate manufacturing process impacts on the use of the many different products available for different applications.
Unique and efficient process
Of its four phosphate production plants in Europe, Tessenderlo Chemie in Ham, northern Belgium, is the biggest, producing more than 50% of the group’s total feed phosphate production. At the Ham plant, the company produces feed-grade phosphates based on HCl produced from the sulphate production process. This unique process was originally developed in 1929 at the original Tessenderlo Chemie factory nearby.
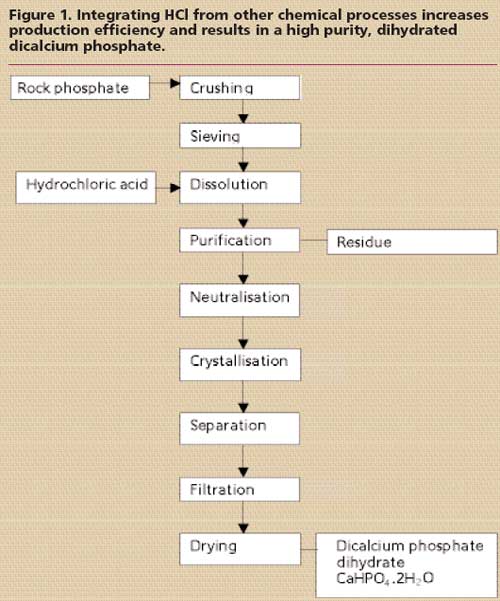
Rock phosphate arrives by boat from North Africa along the King Albert canal, where it is unloaded and stored before being crushed and sieved ready to be purified using the hydrochloric acid dissolution method outlined in Figure 1. Dissolution in HCl results in the formation of a monocalcium phosphate solution. The gradual nature of the dissolution process affords the final product a number of advantages in its use as a feed phosphate. Firstly, as a low temperature process, HCl dissolution avoids the formation of meta- and pyro-phosphates, which are biologically unavailable. Secondly, the crystallisation process reduces variability in composition and improves the purity of the end product, which is virtually free of undesirable contaminants. Finally, the hydrated form of the product is considered to be nutritionally beneficial, due largely to its regular crystal structure. After a counter current purification process, the dihydrated dicalcium phosphate crystals are precipitated, separated, filtered and dried. The crystalline structure is unique to the HCl production process. The dicalcium phosphates is further used for the production of monocalcium phosphate at the factory in Ham.
The HCl process, however, is not the only method by which Tessenderlo’s phosphate products are produced. The “Windmill” range of feed phosphates, produced at the Vlaardingen plant near Rotterdam in the Netherlands, is produced by the so-called “wet acid” route. The rock phosphate is first digested with sulphuric acid, yielding phosphoric acid. After further filtration, purification and defluorination steps, this “feed grade” phosphoric acid is reacted with a calcium source such as quicklime and/or limestone under strictly controlled conditions. Thus, anhydrous dicalcium phosphate (CaHPO4) is formed. Speciality phosphates such as monoammonium- and magnesium phosphates are also produced here. At the group’s plant in Cologna-Veneta, near Verona in Italy, a third range of feed phosphates is manufactured, mainly for the Italian market. The production of Italphos® dicalcium phosphate is based on a similar production principle to the Windmill dicalcium phosphate. By reacting the resulting DCP further with purified feed grade phosphoric acid MDCP (a complex of mono- and dicalcium phosphate) or MCP is produced thereby increasing its phosphorus content further and decreasing its Ca:P ratio.
What’s in a phosphate?
With a variety of different feed phosphate products from the same company apparently competing with each other in an already busy market, it may seem somewhat strange at first glance that Tessenderlo should maintain such a high level of internal competition. However, in doing so, the group is well placed to make a qualified assessment of the quality of different phosphate products for different applications (Table 1), based on their chemical composition. And it has done so extensively, forming a database of the efficacy of different phosphate sources in terms of their phosphorus content, calcium to phosphorus ratio and digestibility in different diets for different species, strains and ages of pigs and poultry.
A major difference between different phosphates is their phosphorus content. Table 2 shows how this can vary between products. Monocalcium phosphate (MCP) produced by enrichment of DCP with defluorinated feed-grade phosphoric acid has the highest phosphorus content on a weight basis. This, say Zwart and Bleukx, is a major reason why many feed manufacturers and producers are beginning to change from DCP to MCP. The increased phosphorus content and high solubility of MCP offer increased flexibility in diet formulation.
Animal trials are the real test
Although the phosphorus content and solubility measurements that can easily be made in the laboratory are a good initial indication of the feeding value of a phosphate source, there is of course, no substitute for digestibility values measured in feeding trials. Table 3 shows the results of independent digestibility trials that have been performed on various different feed phosphates. It is clear from these that the choice of product as defined by its form (monocalcium-, monodicalcium-, dicalcium, calcium-magnesium, magnesium or ammonium form); and the production process used has an effect on the digestibility of the final product and, hence, on the dietary inclusion level required. The data supplied were measured using semi synthetic and practical diets. Way out in front in the digestibility stakes are the monocalcium phosphates for both pigs and poultry. In particular, the MCP derived from DCP produced via the HCl production process has particularly high digestibility because of its purity. The lowest feeding value is afforded by the anhydrous dicalcium phosphates. The second reason for feed manufacturers to change from anhydrous DCP to MCP.











