Clostridium perfringens – a nutritional challenge?
Clostridia are everywhere and different clostridial species are responsible for a number of diseases in poultry. Clostridium perfringens, in particular, is responsible for Necrotic Enteritis. The spores are resistant to high temperatures and chemical treatments, so contaminated feed is a particular problem. So what can we do to prevent it?
Marko Schuring and Bert van Gils
Clostridia are anaerobic bacteria which are ubiquitous and belong to the oldest bacterial species in the world (Kohler, 1992). They are mainly present in the top layers of soils that are rich in organic matter, in dust, water and in the intestinal tract of man and animals. Clostridia are saprophytes, organisms that feed on decaying plant and animal matter. Their role in nature is the breakdown of organic matter and the fixation of inorganic nitrogen.
Numerous noxious Clostridia species exist.
The most dangerous for poultry are:
– C. perfringens
– C. botulinum
– C. colinum
– C. septicum
– C. novyi
– C. sordellii
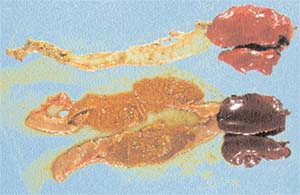
| Intestine and liver from healthy (top) and C. perfringens infected chickens (bottom) |
All the above strains are spore forming and thus resistant to heat and harsh environmental conditions. Most strains survive short-term temperature increases up to 80˚C (Kohler, 1992). Pelleting feed is not sufficient to destroy spores. Even more extreme treatments like extraction of soybeans with hexane and the toasting of the soybean meal, or preparing meat in an oven (Frazier and Westhoff, 1988) are not sufficiently effective to kill all spores. Because of this, feed can be an important source of infection (Kohler, 1992). Clostridia are obligate anaerobic bacteria, which need a low oxygen environment to grow. Contrary to (facultative) aerobic bacteria, Clostridia generate energy by oxygen-free metabolism of amino acids (Kohler, 1992). The formation of organic acids such as butyric acid and acetic acid as well as gas from both carbohydrates and proteins is characteristic. In addition to this, the various strains produce numerous enzymes (lipases, proteinases and urease) as well as endo- and exotoxins. The toxins are mostly enzymes as well, enabling the bacteria to penetrate the tissues of dead bodies and other organic material.
It is by way of these toxins that the Clostridia cause disease. The toxins have necrotic and haemolytic effects and affect the nerves. Clostridia are classified in 5 different types (α to ν), which are characterised by the different (combinations of) toxins they produce. At least 12 different toxins (a till u) have been identified. All toxins produced by Clostridia have protein structures with strong antigenic properties and induce the development of serum anti-toxins and immune resistance.
In poultry, Clostridia can induce several diseases:
- Necrotic Enteritis, an infection of the gut wall that is mainly caused by the α-toxin of C. perfringens type A. This toxin induces necrosis of the intestinal epithelium in the caudal part of the small intestine. It occurs mainly in broilers and geese. It is found more and more in a mild sub-clinical form, which does lead to proliferation of Clostridium in the liver and enhanced condemnations in the slaughterhouse.
- Ulcerative Enteritis, caused by Clostridium colinum. This disease results in lesions from duodenum down to the caeca and spreads via the blood to liver and spleen. A typical symptom is tissue necrosis in combination with local purulent infections. Quails are especially sensitive, but other poultry species are susceptible as well.
- Gangrene, a skin infection with tissue necrosis usually caused by Clostridium perfringens type A, C. septicum, C. novyi type A and/or B and always in combination with aerobic bacteria like Staphylococcus aureus, E. coli or Pseudomonas spp. It is a wound infection that may give rise to gas formation, oedema, bleeding and necrosis.
- Botulism, of which Clostridium botulinum type C is the causative agent. It is an intoxication with type C neurotoxin. The toxin induces paralysis, especially of the wings, the legs and the neck. The disease is more common during the summer than during the winter.
More than 89% of the cases of Clostridium problems in broilers are caused by C. perfringens, type A (Kohler, 1992). Type C is responsible for less than 3% of the cases. Both strains produce the α-toxin, to which broilers are particularly sensitive.
Necrotic Enteritis (NE)
The direct causative agent of NE is C. perfringens type A or C in particular Particularly the –toxin produced by both types of C. perfringens, however the α-toxin produced by C. perfringens type C are also responsible for the tissue necrosis in the gut (Songer, 1996). C. perfringens can be isolated by incubation of blood agar plates during 1 night at 37°C. By incubation in different substrates a final identification can be made. Most strains ferment glucose, maltose, lactose and sucrose, producing butyric acid and acetic acid, but do not ferment mannitol.
Outbreaks of Necrotic Enteritis have been observed in poultry from 2 weeks through to 6 months of age. Usually NE occurs in broilers between 2 and 5 weeks of age housed on litter. Occasionally cases have been reported in laying hens in floor-keeping systems and in pullets in batteries. In the field, typical clinical outbreaks with high mortality rates are very rare. Usually the disease has a sub-clinical course without clear specific symptoms.
C. perfringens spp. are found in litter, soil, dust, and contaminated feed as well as in the intestinal contents. Several factors are known to predispose or relate to Necrotic Enteritis:
- Contaminated feed or litter
- Feed composition
- Anti-microbial growth promoters and coccidiostats
- Other infections of the intestinal tract, such as coccidiosis, malabsorption syndrome, dysbacteriosis and mycotoxicosis
- Immune-suppressive agents like IBD (infectious bursal disease), CAA (Chicken anaemia virus) and Marek’s disease, but also non-specific stress
- The genetic constitution of the modern broiler, which is geared towards growth rate and feed efficiency, but which possibly has a less well-developed immune defence system.
Clinical picture and lesions
When broilers suffer from an outbreak of NE, the feed intake and growth rates are substantially depressed. They mostly sit, have loose droppings and a rough feather coat. In acute outbreaks the clinical symptoms are only observed very briefly. Birds die within a short period of time after the infection.
At necropsy the lesions are usually restricted to the middle and caudal part of the small intestine. The intestinal epithelium is brittle and swollen due to gas formation, the mucous layer is often greenish yellow, but bleeding is rare. Occasionally there are lesions in the caeca and in the liver.
Microscopic examinations reveal severe necrosis of the mucosal layer with abundant cellular debris. At the onset of an infection, the tips of the villi are damaged, causing death of the epithelial tissues. At the site of the lesions clostridia colonise the lower tissue layers causing tissue necrosis. Even the muscular layers of the gut wall can be infected. After healing the new villi are short and malformed resulting in an impaired absorptive capability.
Diagnosis is based on the clinical picture, visible lesions of the gut wall, microscopic analysis and bacteriological tests to isolate Clostridia from gut samples. Some other diseases have symptoms similar to Necrotic Enteritis:
- Ulcerative Enteritis caused by C. colinum. This disease however also causes necrosis of the liver and caeca.
- An infection with Eimeria brunetti. Laboratory analyses are needed to discriminate between the two. NE and coccidiosis occur often simultaneously, coccidiosis being one of the predisposing factors for NE.
Necrotic enteritis can be treated effectively with several antibiotics. It is recommendable to consider simultaneous treatment against coccidiosis as well.
Effect of AGP’s and coccidiostats
Several antibiotics can be effectively used as a drug against C. perfringens. Lincomycin, Bacitracin, Oxytetracyclin, Penicillin and Tylosin are some of these products. A number of anti-microbial growth promoters have been shown to have a direct effect on C. perfringens. Zinc bacitracin, Virginiamycin, Avoparcin and Avilamycin belong in this category.
As a consequence of legislation, all antibiotic growth promoters have disappeared from the European market since 1 July 1999, except Avilamycin and Flavophospholipol. It is not impossible that a general ban on antibiotic growth promoters will follow within 3 to 4 years. The feed manufacturer has a limited number of options to control Clostridia by feed additives.
1. anti-microbial growth promoter
2. coccidiostats
3. live bacterial cultures
4. essential oils
- Anti-microbial growth promoters – As mentioned before, only two options are left for the near future, Avilamycin and Flavophospholipol. At a dose level of 10 ppm, costs of both products are comparable. In 1993 and 1998, Devrieze measured MIC values of several antibiotics for Clostridia. In 1993 and 1995, different strains of Clostridia were tested and 12 in 1998. The results are reported in Table 1. From Table 1 it appears that Avoparcin and Avilamycin have particularly low MIC values for Clostridia. It also appears that Flavophospholipol has no direct effect against Clostridium species. The MIC value for Bacitracin has substantially deteriorated
- over a period of 5 years, although the smaller number of Clostridia strains in the test may be partially responsible for this observation.
- Coccidiostats – Some anticoccidial agents have a direct bactericidal effect on C. Perfringens. Relative to common dose levels in the feed the MIC values of Narasin and Salinomycin are very good. Narasin is reported to be very effective against Clostridia problems in broiler flocks (Elwinger et al., 1992). Because of the fact that coccidiosis is an important predisposing factor in the occurrence of Clostridium (Kohler, 1992) all effective coccidiostats will have a favourable effect on Clostridium via the suppression of coccidiosis.
- Bacterial cultures – A balanced and stable gut flora is an important barrier to Clostridia. Songer (1996) reports experiences where germ-free chicks had very high mortality rates (50%) after a challenge with C. perfringens, whilst chicks inoculated with Lactobacillus acidophilus or Enterococcus faecalis had very low mortality. Conventional chicks had no mortality at all in similar conditions. The application of Aviguard (Hofacre et al., 1998) or Broilact (Elwinger et al., 1992) to day-old chicks is effective in reducing the number of Clostridia in the caeca.
- Essential oils – Essential oils are claimed to have favourable effects on Necrotic Enteritis (Kohler, 1999). The effectiveness is said to be at least similar to the effect of Bacitracin. The latter, however, appeared to have a doubtful MIC in the most recent tests conducted by De Vrieze (1998).
Other preventive measures
Kohler (1999) has demonstrated that the severity of a Clostridium infection is linearly related to the number of spores per gram of feed. Besides the litter, feed is a very important route of infection. Due to the fact that Clostridium spores are inert to chemical and mechanical treatments, it is practically infeasible to produce feed that is free of Clostridium spores. Normal preconditioning and pelleting of the feed is not sufficient.
No research to the effect of ring-dye expansion or extrusion is available. Even more severe hydro-physical or chemical treatments of feed will certainly destroy important nutrients. However, general hygienic measures with regard to feed hygiene should help to keep the infection level as low as possible. Measures in this category will show a strong similarity to those taken in the context of Salmonella control (control of vermin, birds and insects).
Clostridial problems usually occur as secondary infection with diseases like coccidiosis, MAS and dysbacteriosis. The bacterial overgrowth of the small intestine (dysbacteriosis) creates gut conditions with a low oxygen pressure, favourable for Clostridia. Some claim that dysbacteriosis is the actual problem, Clostridium being no more than a secondary consequence (Fabri 1999, oral communication). Solving the dysbacteriosis problem might solve the Clostridium problem as well.
Therefore all nutritional measures which support a stable intestinal flora and which have a preventive action against dysbacteriosis, malabsorption or coccidiosis, may be effective barriers for Clostridium as well. Although Flavomycin has no direct effect against Clostridia, it is claimed that it is very effective against Clostridia due to its positive effect stabilising the gut flora (Tange, L., 1999, oral communication). It is very well possible that other feed additives have such an indirect action as well and will prove to be effective tools in the fight against Clostridia.
Other nutritional measures generally recommended to alleviate the symptoms of or prevent malabsorption syndrome or dysbacteriosis include:
· Highly digestible ingredients
– corn
– enzymes if wheat, barley, triticale or other pentosan-rich cereals are used
– vegetable oils instead of tallow
· Minimisation of the amount of indigestible protein, which give rise to proteolytic fermentation
– Use of good quality proteins (especially important in case of meat and bone meal and fish meal)
– Limitation of the crude protein content of the feeds by smart use of synthetic amino acids.
· Avoidance of raw materials contaminated with Clostridium spores, e.g. flattened cereals and peas harvested under poor weather conditions
The above recommendations are based on field experiences. Unfortunately due to the lack of good research models, no hard scientific data are available to support them.
References
Benno, Y et al. (1988) Jpn Vet. Sci. 50 (3) 832-834
Devriese, L.A et al. (1993) J.Applied Bacteriology 75, 55-57
Elwinger, Ket al. (1992) Acta vet. scand. 33, 369-378
Frazier, W.M.C.and Westhoff, D.C. (1988) Food Microbiology, fourth edition, McGraw-Hill Book Company
Hofacre, Ch.L et al. (1998) J. Appl. Poultry Res.7, 412-418
Kohler, B. (1992). In: Krankheiten des Wirstschaftsgeflugel, ein Handbuch fur Wissenschaft und Praxis, Gustav Fischer Verlag Jena, Stuttgart 1992, 195-225
Kohler, B. (1999). In: Animal Feed and AKZO NOBEL, AKZO NOBEL symposium, 10 March , Rosmalen
Songer, J.G. (1996). Clinical Microbiological
Reviews 9 (2) 216-234











